TIME-TO-MARKET
STARTUP WITH RAPID
TECHNOLOGICAL ADVANCEMENTS
DEVELOPMENT CHALLENGES
AND CERTIFICATION FOR
MARKET SUCCESS
& RESOURCE ALLOCATION
DEVELOPMENT CHALLENGES
AND CERTIFICATION FOR
MARKET SUCCESS
MANAGEMENT & RESOURCE
ALLOCATION
MEDTECH STARTUP WITH
RAPID TECHNOLOGICAL
ADVANCEMENTS
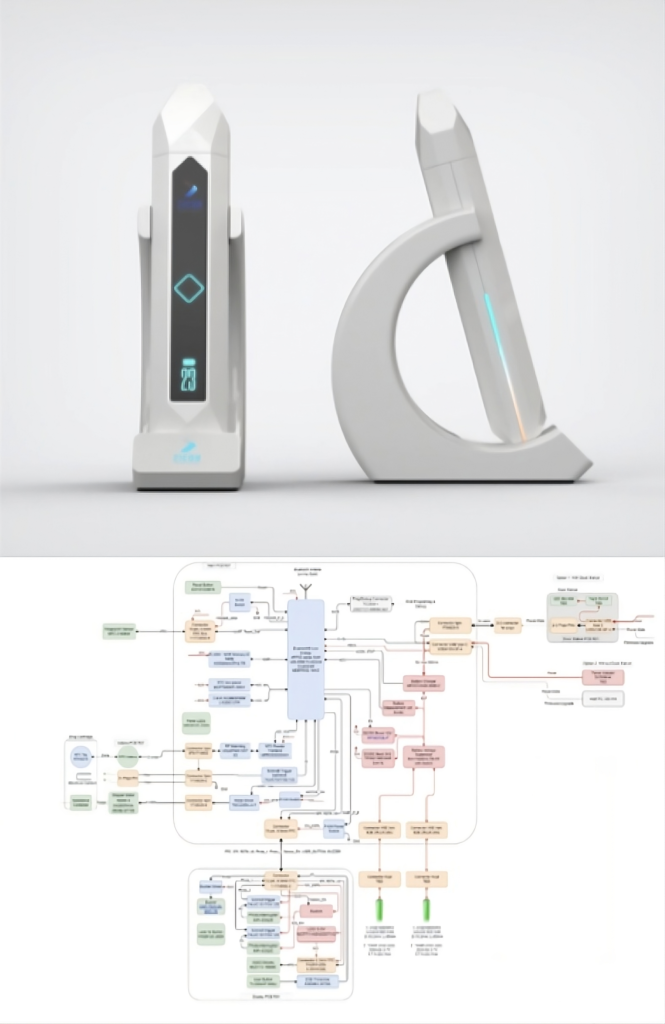
Drug Delivery System
Transforming Healthcare & MedTech Through Innovation and Expertise
Access to Specialized Expertise
Our team includes seasoned professionals with extensive experience in medical device development, ensuring top-tier expertise across all phases of your project.
Cost Efficiency
Outsourcing to TechFlex-Dev reduces the need for significant capital investment in specialized equipment and personnel, resulting in substantial cost savings.
Regulatory and Compliance Excellence
With a robust understanding of FDA, ISO, and other regulatory requirements, we ensure that your products meet all necessary compliance standards, minimizing risks and delays.
Cutting-Edge Technology Integration
We leverage the latest advancements in IoT, AI, and digital health, ensuring your products are innovative and future-proof.
Proven Track Record
Our history of successful projects and satisfied clients demonstrates our capability to handle complex development challenges effectively.
Comprehensive Development Solutions
for MedTech
"End-to-End Hardware and Software Development Solutions for Medical Devices"
Hardware Development
Advanced Engineering
Our team excels in the design and development of advanced medical device hardware, including sensors, PCBs, and durable enclosures tailored to meet stringent industry standards.
Rapid Prototyping
We utilize state-of-the-art technologies to create functional prototypes quickly, enabling thorough testing and validation to meet regulatory requirements.
Production Support
From concept to production, we provide comprehensive support to ensure your medical devices are market-ready and compliant with FDA and ISO standards.
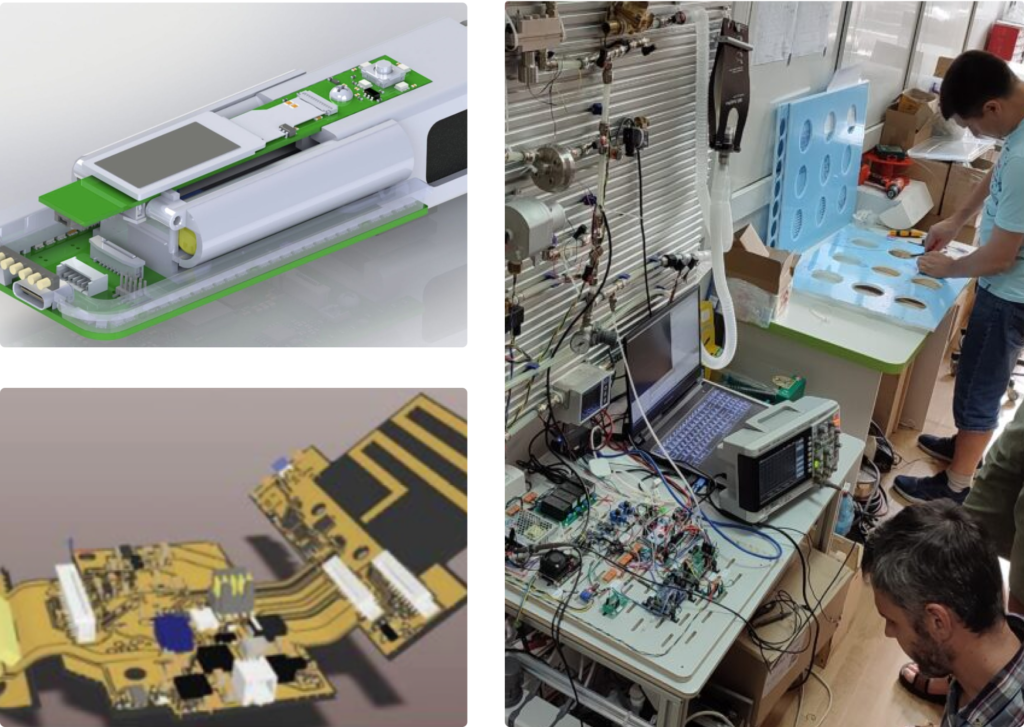
Custom Software Development

Custom Medical Software
We develop bespoke software solutions for medical devices, including embedded systems, patient management applications, and cloud-based data platforms.
System Integration
Ensuring seamless integration with existing healthcare IT systems, such as EHR/EMR platforms, to enhance interoperability and streamline workflows.
Compliance and Security
Our software solutions adhere to HIPAA regulations, ensuring data privacy and security, and meet CE and FDA guidelines for medical software.
Why Growing Companies and Startups Should Use TechFlex-Dev's Outsourcing Team
Addressing Key Pain Points for Early-Stage and Series A Startups
- Pain Point: Startups often lack the extensive resources required for comprehensive medical device development.
- Solution: TechFlex-Dev provides access to a multidisciplinary team of seasoned professionals, reducing the need for significant capital investment in specialized equipment and personnel.
- Pain Point: Managing budget constraints while striving to develop high-quality products can be a significant challenge.
- Solution: Our dual-location strategy leverages cost-effective development teams in Eastern Europe, optimizing your budget without compromising on quality. This approach allows startups to allocate their funds more efficiently and invest in critical areas of growth.
- Pain Point: Accelerating time-to-market is crucial for gaining a competitive edge and achieving rapid regulatory approval.
- Solution: TechFlex-Dev streamlines the development process from concept to commercialization, utilizing efficient workflows and expert project management to reduce time-to-market. Our rapid prototyping capabilities ensure thorough testing and validation, meeting regulatory requirements swiftly.
- Pain Point: Navigating the complex landscape of regulatory compliance can be daunting for startups.
- Solution: With over two decades of experience, our team ensures that your products meet all necessary compliance standards, including FDA, ISO, and CE guidelines. This minimizes risks and delays, providing peace of mind and a clear path to market.
- Pain Point: Startups may lack access to specialized expertise in medical device development.
- Solution: Our team includes experts in advanced engineering, software development, and regulatory compliance. This comprehensive expertise ensures that your projects are handled with the utmost professionalism and technical proficiency.
Addressing Key Pain Points for Early-Stage and Series A Startups
Limited Resources
MedTech Startups often lack the extensive resources required for comprehensive medical device development.
TechFlex-Dev provides access to a multidisciplinary team of seasoned professionals, reducing the need for significant capital investment in specialized equipment and personnel.
Budget Constraints
Managing budget constraints while striving to develop high-quality products can be a significant challenge.
Our dual-location strategy leverages cost-effective development teams in Eastern Europe, optimizing your budget without compromising on quality. This approach allows startups to allocate their funds more efficiently and invest in critical areas of growth.
Time-to-Market Pressures
Accelerating time-to-market is crucial for gaining a competitive edge and achieving rapid regulatory approval.
TechFlex-Dev streamlines the development process from concept to commercialization, utilizing efficient workflows and expert project management to reduce time-to-market. Our rapid prototyping capabilities ensure thorough testing and validation, meeting regulatory requirements swiftly.
Regulatory Compliance
Navigating the complex landscape of regulatory compliance can be daunting for startups.
With over two decades of experience, our team ensures that your products meet all necessary compliance standards, including FDA, ISO, and CE guidelines. This minimizes risks and delays, providing peace of mind and a clear path to market.
Access to Expertise
Startups in the MedTech Industry may lack access to specialized expertise in medical device development.
Our team includes experts in advanced engineering, software development, and regulatory compliance. This comprehensive expertise ensures that your projects are handled with the utmost professionalism and technical proficiency.
Lung Ventilation System
TechFlex Team

Jorg Lorscheider
CEO

Shepard Bentley
VP Regulatory Affairs
25+ years quality management and regulatory consulting.
Our Success Stories
TechFlex


Blog And Resources
Insights and Resources
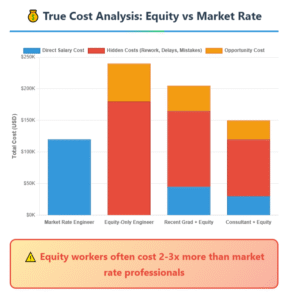
Exit Strategy Reality: Who’s Actually Buying Medical Device Companies?
“The cheapest team member might be the most expensive mistake you make.

MVP or Bust: Cutting Features to Save Your Company
“The cheapest team member might be the most expensive mistake you make.

Francis Medical gets FDA green light for water vapor ablation to treat prostate cancer

Francis Medical garnered a 510(k) clearance for its water vapor ablation therapy for cancers of the prostate, kidneys and bladder.
Read the full article at: www.fiercebiotech.com

Joint Preservation Innovations Announces FDA 510(k) Clearance for the Articulator™ Arthroscopic Bur — the First and Only FDA-Cleared Articulating Rotary Cutting Instrument

Joint Preservation Innovations Inc. announced that FDA has granted 510(k) clearance for the Articulator™ Arthroscopic Bur.
Read the full article at: ryortho.com

FDA making use of real-world evidence in device applications easier (BSX:NYSE)

Learn how the FDA is easing real-world evidence rules for medical devices, reducing data burdens for sponsors.
Read the full article at: seekingalpha.com
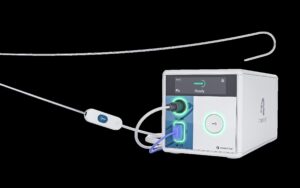
Atraverse Medical Secures FDA 510(k) Clearance for HOTWIRE™ RF Generator & Fully Integrated Transseptal Access System
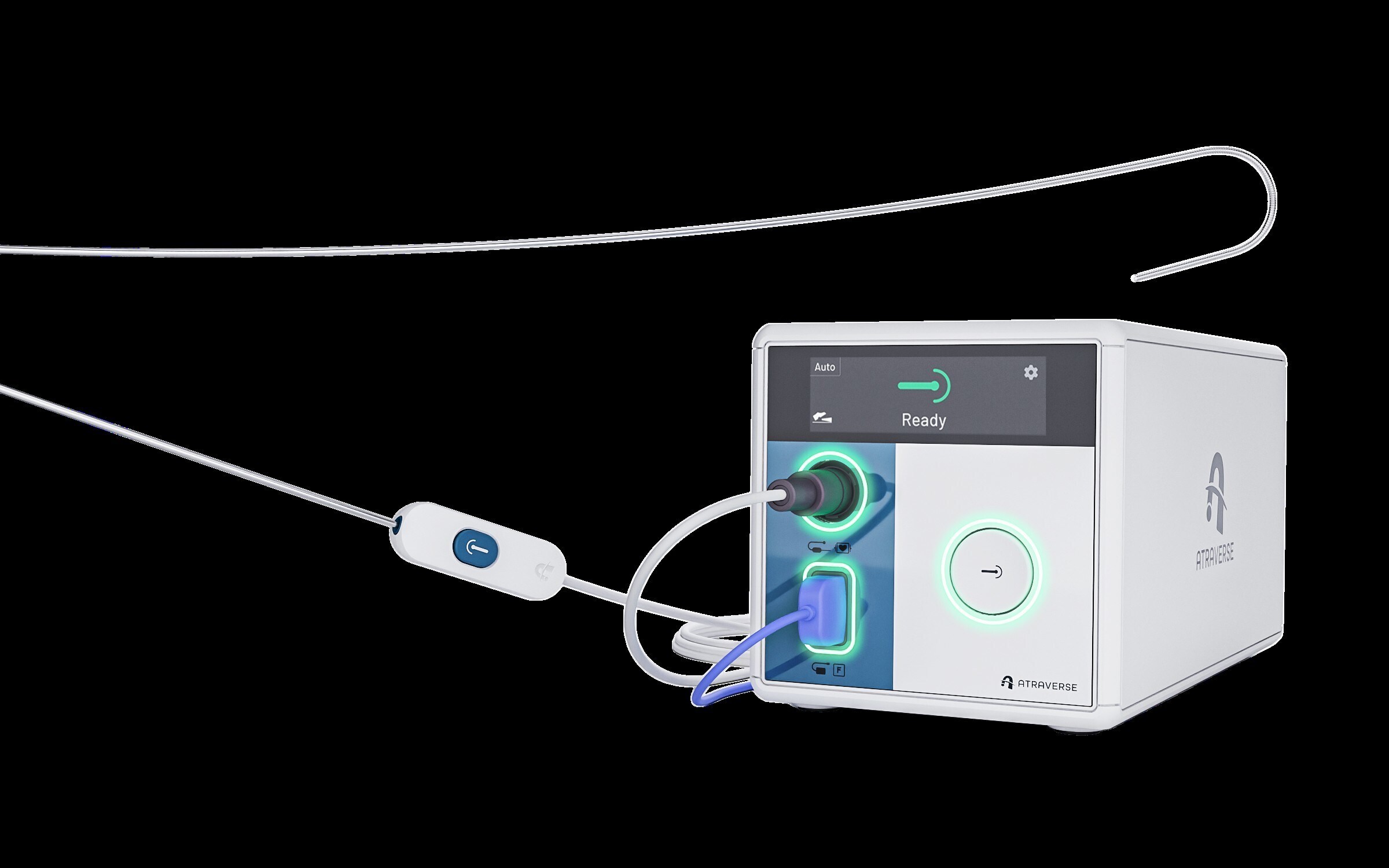
/PRNewswire/ — Atraverse Medical, a medical device company pioneering next-generation left-heart access technology, has received 510(k) clearance from the U.S….
Read the full article at: www.prnewswire.com




