Accelerate Your MedTech Innovation with TechFlex
The MedTech industry is characterized by rapid advancements, evolving technologies, and stringent regulatory requirements. To stay ahead of the competition, companies must innovate quickly while ensuring that their products are safe, effective, and compliant with all regulations. However, accelerating innovation in MedTech can be challenging due to the complexity of medical devices, the need for rigorous testing, and the extensive approval processes required. Companies often struggle with balancing speed and quality, leading to delays in bringing innovative products to market.
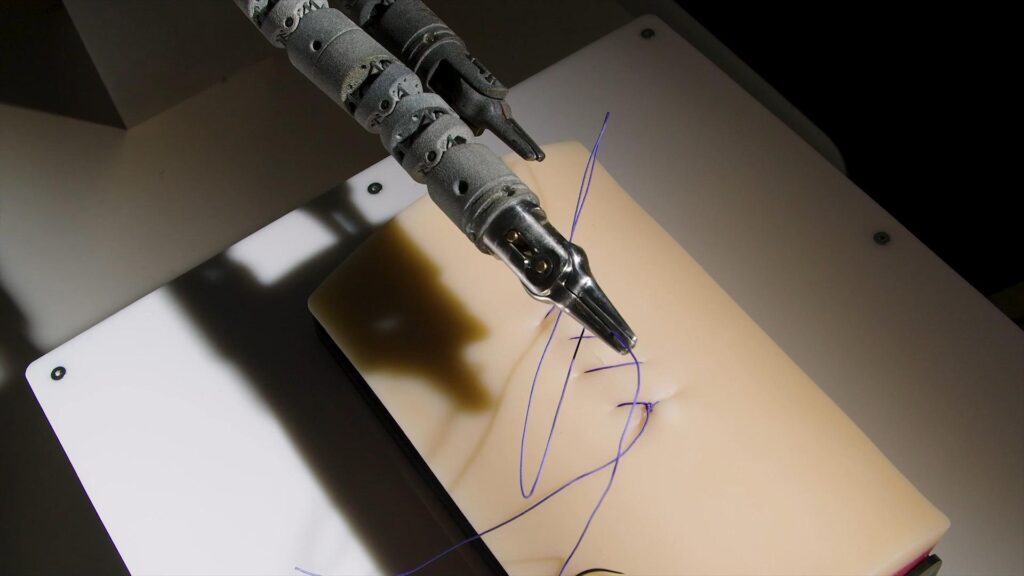
How TechFlex Solves This Problem
TechFlex is dedicated to helping MedTech companies accelerate their innovation processes without compromising on quality or compliance. Our comprehensive suite of services—ranging from cutting-edge software and hardware development to in-depth regulatory expertise—ensures that your MedTech innovations are developed efficiently and effectively, allowing you to bring new products to market faster.
Key Solutions Offered by TechFlex
Cutting-Edge Research and Development
➣Advanced Prototyping:
TechFlex leverages state-of-the-art prototyping techniques to quickly transform your innovative ideas into functional prototypes. Our rapid prototyping capabilities allow you to test and refine your concepts early in the development process, reducing time spent on revisions and accelerating the overall timeline.
➣Innovative Technologies:
We utilize the latest technologies, such as AI-driven analytics, IoT integration, and advanced prototyping techniques, to enhance your MedTech innovations. These technologies not only improve the functionality and reliability of your products but also speed up the development process.
Streamlined Development Processes
➣ Agile Methodology:
Our Agile development methodology is designed to keep your project moving forward quickly and efficiently. By working in iterative cycles with continuous feedback loops, we ensure that your product evolves rapidly while remaining aligned with your goals and regulatory requirements.
➣ Integrated Development Teams:
TechFlex employs integrated teams of software developers, hardware engineers, and regulatory experts who work together seamlessly. This collaborative approach ensures that all aspects of your MedTech innovation are developed in parallel, reducing bottlenecks and speeding up the overall process.
Regulatory Compliance Expertise
➣ Proactive Compliance Strategy:
Navigating the complex regulatory landscape is critical to accelerating MedTech innovation. TechFlex’s in-house regulatory experts work closely with your team from the outset, developing a proactive compliance strategy that addresses all relevant standards and regulations, such as FDA and MDR. This approach ensures that regulatory hurdles are identified and addressed early, preventing delays during the approval process.
➣ Comprehensive Documentation:
We provide comprehensive documentation and support throughout the development process, ensuring that your product meets all regulatory requirements and is fully prepared for submission to regulatory bodies.
Efficient Testing and Validation
➣ Rigorous Testing Protocols:
TechFlex implements rigorous testing protocols throughout the development lifecycle, including functional, performance, and safety testing. By identifying and resolving issues early, we ensure that your product meets all necessary standards and is ready for market entry sooner.
➣Accelerated Validation Processes:
Our accelerated validation processes are designed to streamline the testing and approval stages, allowing you to move from development to market more quickly. We work closely with regulatory agencies to facilitate fast-tracked approval pathways wherever possible.
Outsourcing Benefits with TechFlex
Outsourcing your MedTech innovation to TechFlex offers numerous benefits that directly contribute to faster development and market entry:
- Access to Cutting-Edge Expertise: By outsourcing to TechFlex, you gain access to a team of experts who are at the forefront of MedTech innovation. Our specialized knowledge and experience enable us to tackle even the most complex challenges, ensuring that your product is both innovative and compliant.
- Cost-Effective Development: Developing innovative MedTech products in-house can be costly and time-consuming. TechFlex offers cost-effective solutions by leveraging our existing infrastructure and expert teams, allowing you to focus your resources on other critical areas of your business.
- Faster Time-to-Market: Our streamlined development processes, combined with our expertise in rapid prototyping, Agile methodology, and regulatory compliance, ensure that your product reaches the market faster. This accelerated time-to-market gives you a competitive edge in the fast-moving MedTech industry.
- Focus on Core Competencies: With TechFlex managing the complexities of MedTech innovation, your internal team can focus on core business activities such as product strategy, customer engagement, and sales, further driving your company’s growth and success.
Confidently navigate the challenges of MedTech development, secure in the knowledge that you have a capable and experienced partner by your side.



