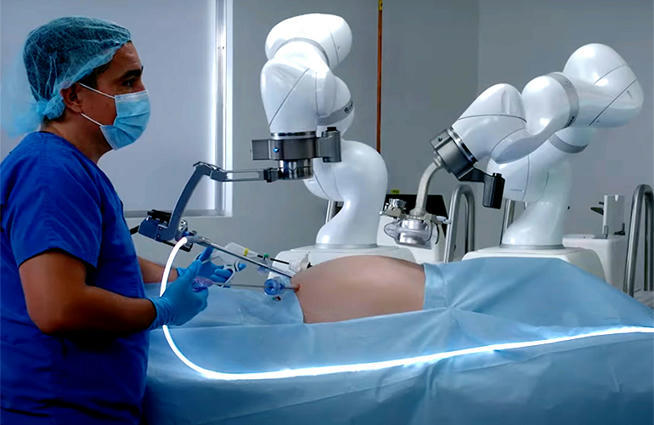
Levita Magnetics announced today that it received expanded FDA 510(k) clearance for its MARS (magnetic-assisted robotic surgery) system.
Read the full article at: www.massdevice.com

Levita Magnetics announced today that it received expanded FDA 510(k) clearance for its MARS (magnetic-assisted robotic surgery) system.
Read the full article at: www.massdevice.com