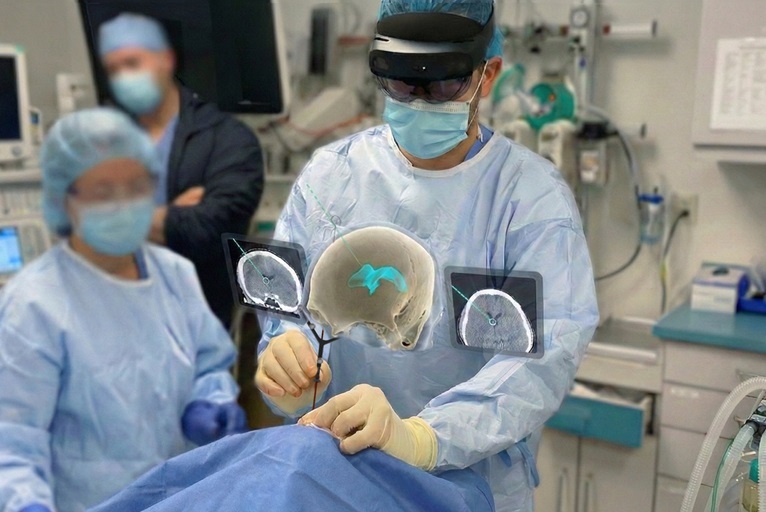
Medivis has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance for its cranial navigation platform, making it the “world’s first” augmented reality (AR) system cleared for intraoperative guidance in cranial neurosurgery, according to the company.
Read the full article at: neuronewsinternational.com


