Resources/ Blogs
TechFlex Team
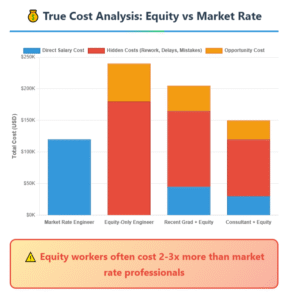
This combined approach allows TechFlex-Dev to offer cost-effective solutions without compromising on quality. By minimizing the need for heavy capital investments in specialized equipment and personnel, we help manage your budget more effectively. Our development processes are meticulously designed to align with regulatory standards, ensuring your medical devices are compliant, reliable, and market-ready.

Jorg Lorscheider
CEO
25 years experience leading engineering service companies.

Shepard Bentley
VP Regulatory Affairs
25+ years quality management and regulatory consulting.
TechFlex-Dev optimizes cost management by leveraging a dual-location strategy. Our project management and account leadership teams are based in the USA, providing seamless communication, alignment with your time zone, and strategic oversight. This ensures that your projects are managed efficiently and meet all regulatory standards, including FDA and ISO requirements.
Meanwhile, our highly skilled development teams operate out of several locations in Eastern Europe. This strategic distribution allows us to tap into a pool of top-tier engineering and development talent at significantly lower costs. Eastern Europe offers a competitive pricing structure due to lower operational costs while maintaining high performance and quality standards.