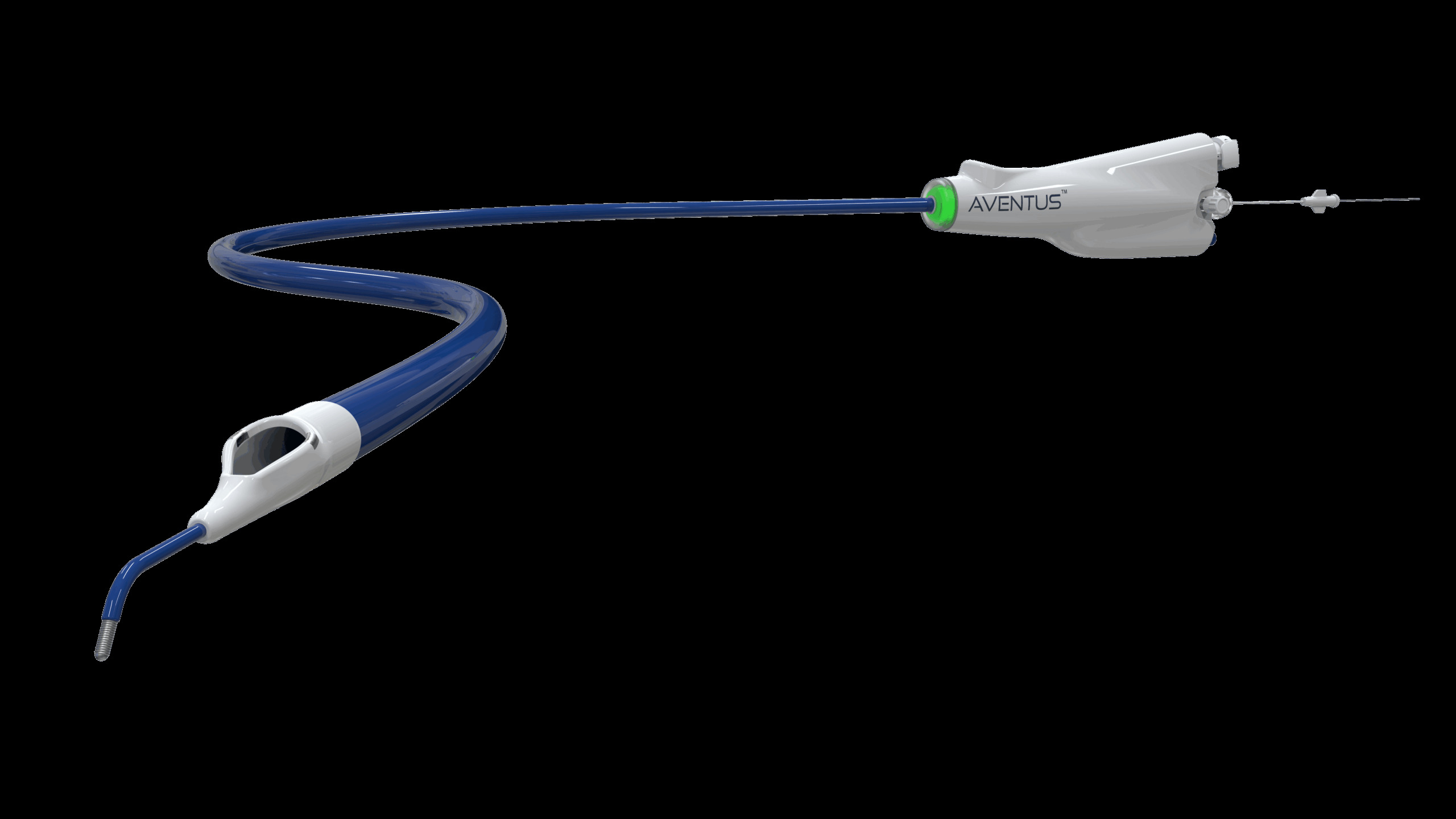
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).
Read the full article at: vascularnews.com

Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).
Read the full article at: vascularnews.com